SFJ Philosophy
SFJ’s decision-making process aims at enhancing the probability of clinical and regulatory success and maximizing the future value of the asset. Decisions are made in collaboration with the sponsor. A few examples:
- Study A – Within the first 9 months of the study, sponsor’s plan was well behind enrollment timelines. SFJ rapidly intervened with additional funding and a plan to increase site numbers. The study ultimately finished 3 months ahead of schedule.
- Study B – SFJ dramatically revised inclusion criteria and resized the sponsor’s trial significantly. These changes ensured focus on the appropriate patients and enhanced likelihood of clinical success. A parallel trial by the sponsor in a different indication failed, while SFJ’s trial led to the drug’s approval.
- Study C – SFJ refined patient inclusion criteria and reclassified endpoint sequence. Trial success was credited in part to these changes.
Case Study:
Vizimpro Co-Development With Pfizer
In 2012, SFJ began a partnership with Pfizer for the funding, design, and execution of a 452-patient global Phase 3 trial of dacomitinib (Vizimpro), a first line therapy for EGFR-mutated non small cell lung cancer. Upon the success of the trial, this once-daily oral medication received regulatory approvals globally between December 2018 and May 2019.
Process Highlights For This Global Registration Trial:
- SFJ and Pfizer agreed basic study design parameters
- SFJ assembled a world-class team of KOL’s as its Scientific Advisory Committee, with representation from Hong Kong, China, Japan, Europe and the US
- SFJ’s team led the protocol-drafting effort, with regular alignment meetings with Pfizer peers
Results: Several Success-Enhancing Design Changes
- Inclusion criteria adjustments
- Exclusion criteria adjustments
- Significant increase in the number of subjects to enhance trial powering
- Operational excellence and efficiency:
SFJ Value-Add
Within 9 months of the Co-Development Agreement closing, SFJ:

- Drafted the protocol and all study documents.
- Completed all required regulatory meetings.
- Selected a CRO
- Secured the comparator drug supply
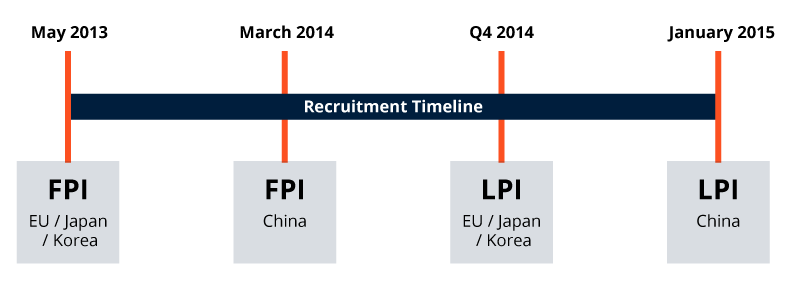
SFJ Accelerated Recruitment Around The Globe:
Efficiency From Database Lock To NDA